
Introduction Sodium bicarbonate, NaHCO3 (MW 84.007 g/mol), is commonly known as baking soda. Sodium - brainly.com
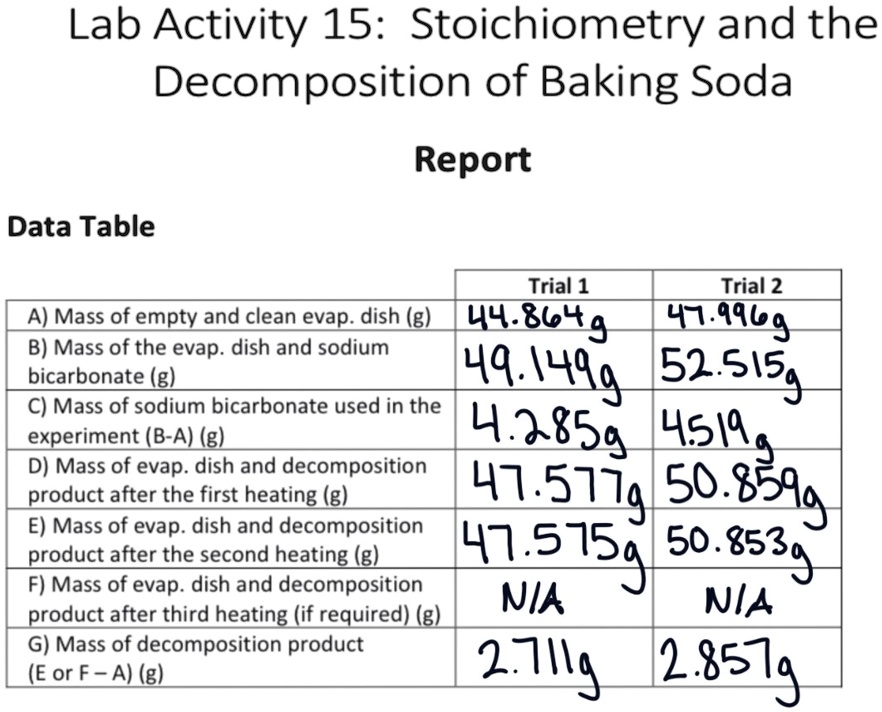
SOLVED: Lab Activity 15: Stoichiometry and the Decomposition of Baking Soda Report Data Table Trial 1 Trial 2 A) Mass of empty and clean evap. dish (g) 44.8649 47.9969 B) Mass of

stoichiometry - During the decomposition of sodium bicarbonate lab, the mass of the final solid I received was less than expected. Errors? - Chemistry Stack Exchange

ol ! CUM .: K, has no unit. equili Problem 12.4 : Write the equilibrium constant expression the decomposition of baking soda. Deduce the unit of K from the above expression. Solution :





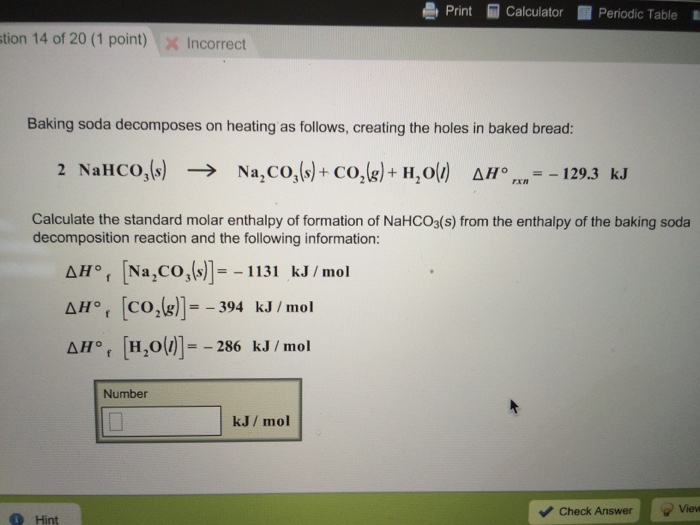


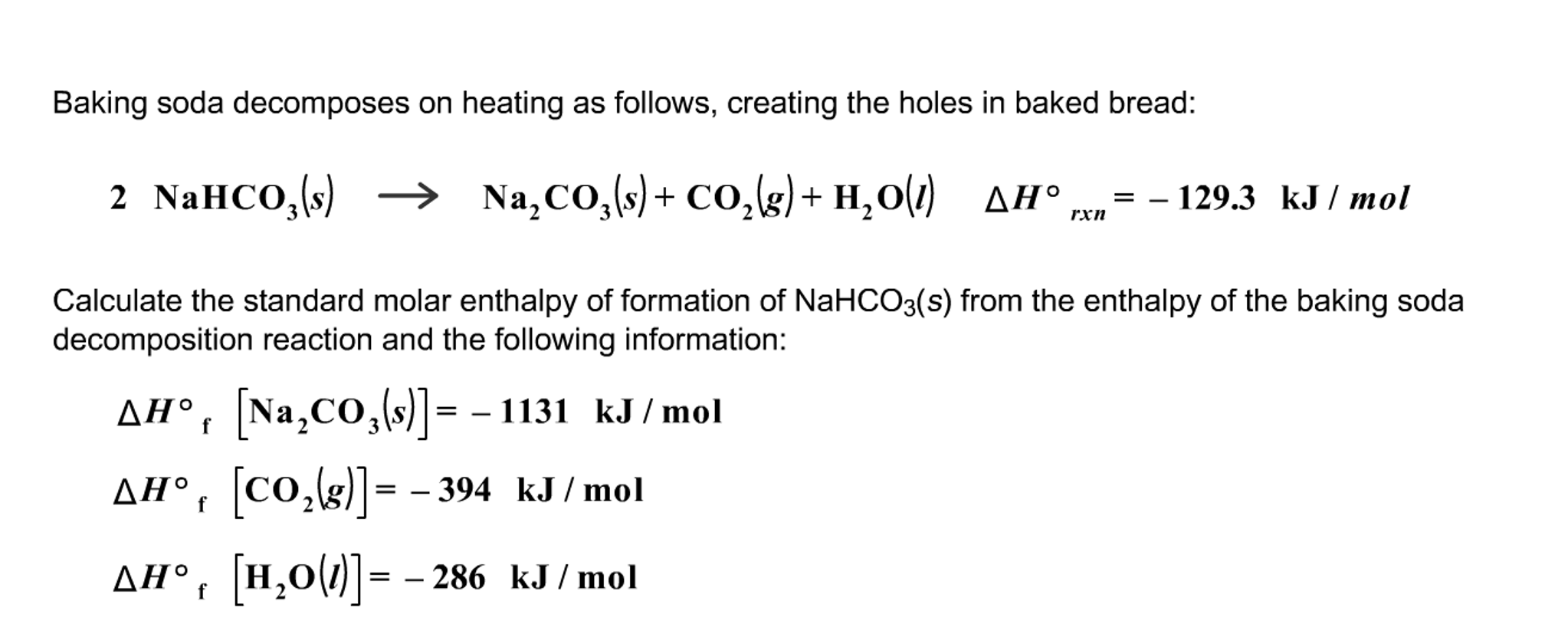


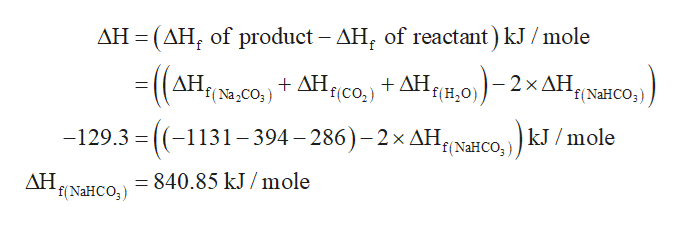
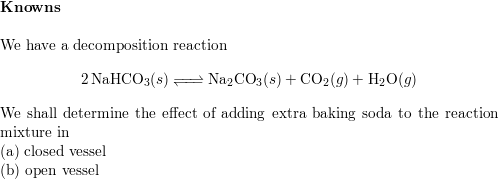

:max_bytes(150000):strip_icc()/185329704-56a1300e5f9b58b7d0bce3ce.jpg)
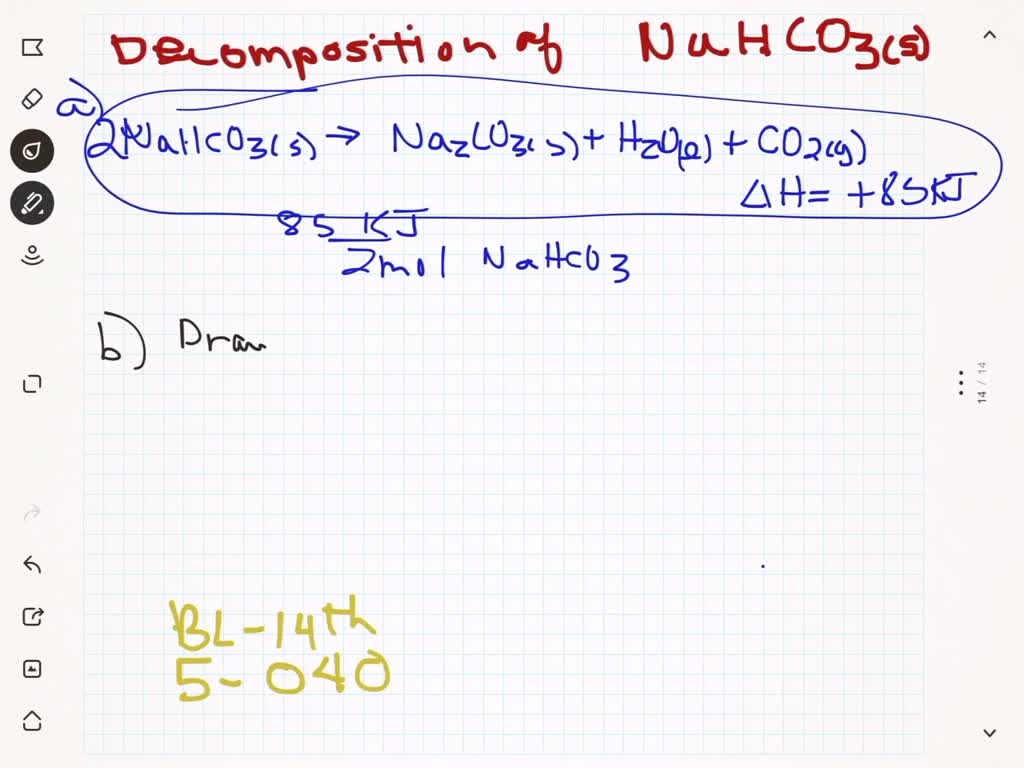
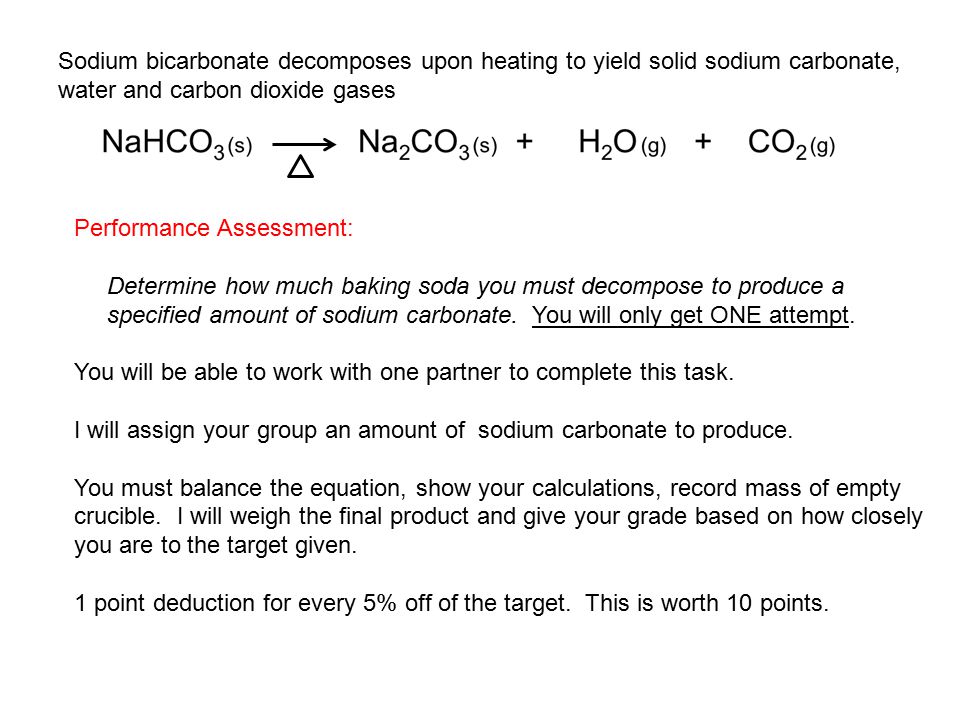
:max_bytes(150000):strip_icc()/sodiumbicarbonate2-599f0a4cb501e800113dd78f.png)