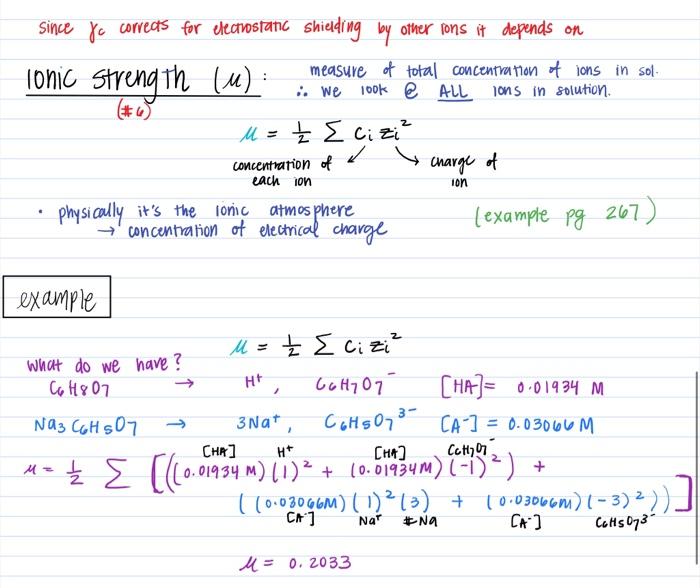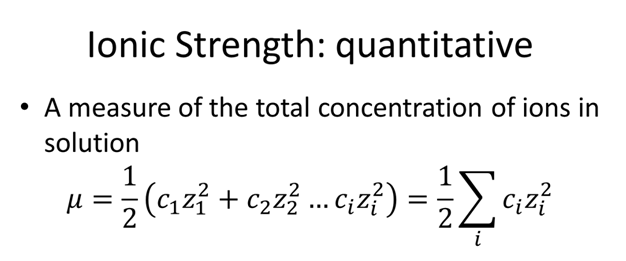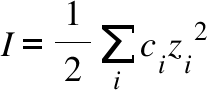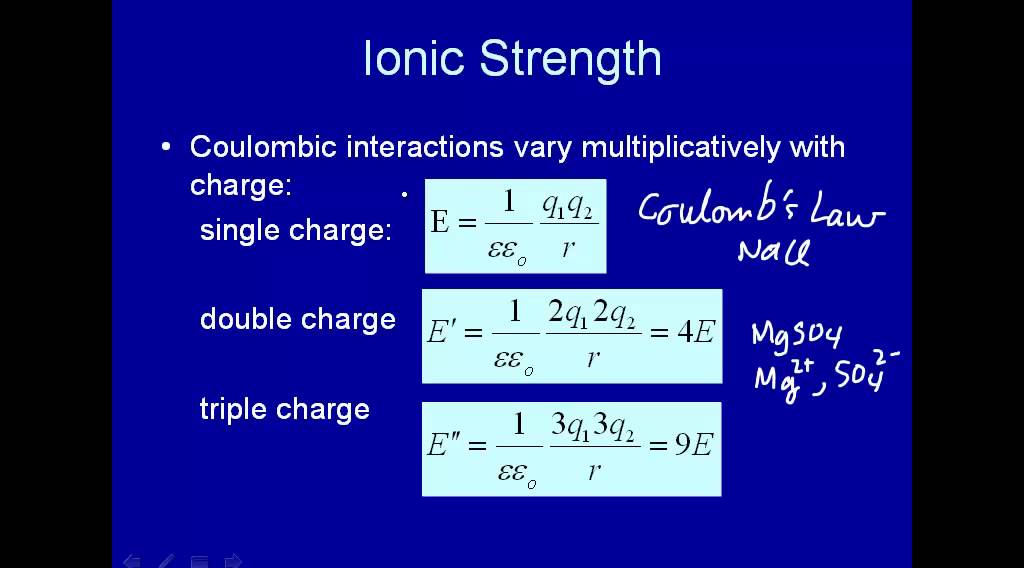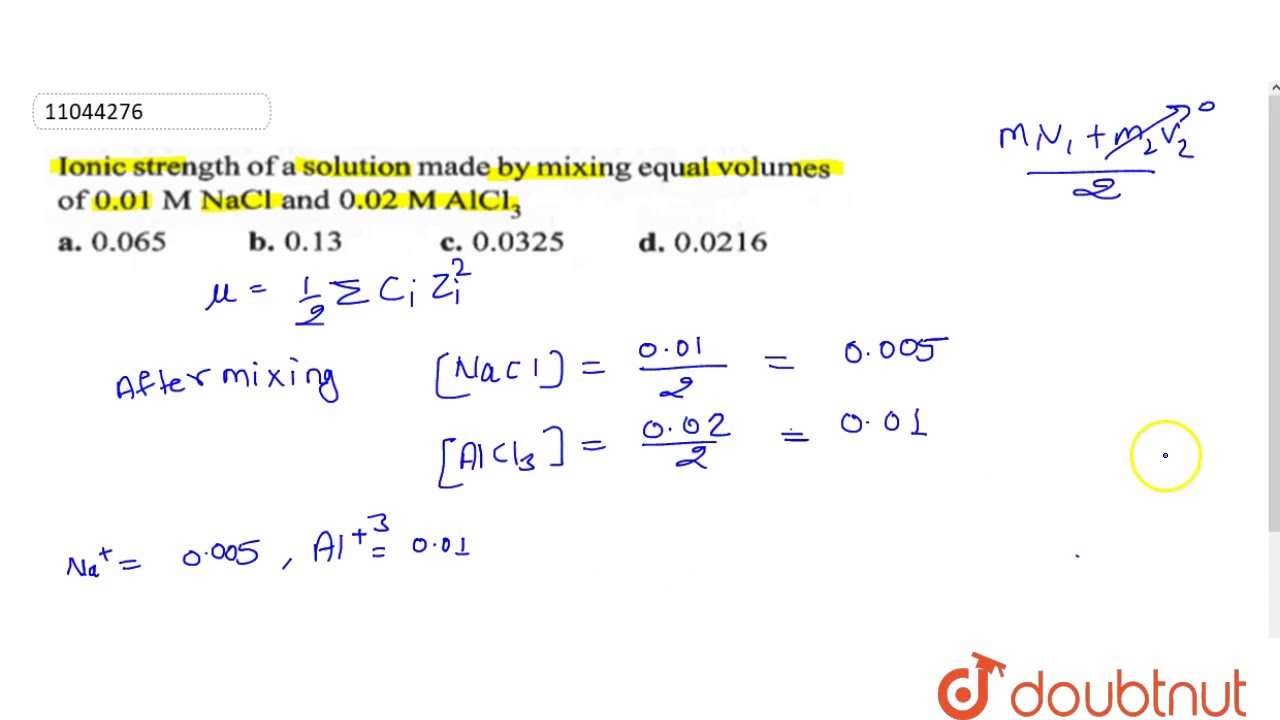
Ionic strength of a solution made by mixing equal volumes of `0.01 M NaCl` and `0.02 M AlCl_(3)` - YouTube
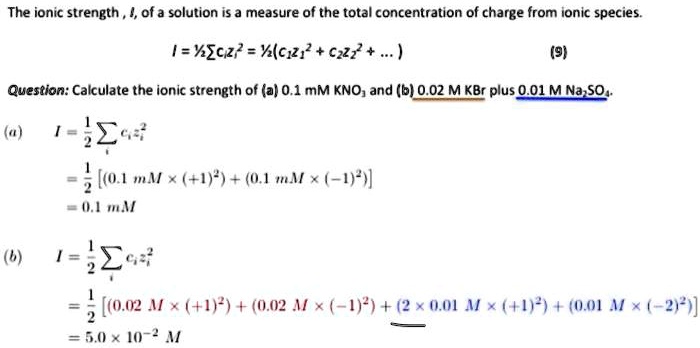
SOLVED: The ionic strength of a solution is a measure of the total concentration of charge from ionic species. (a) Calculate the ionic strength of 0.1 mM KNO3: I = (0.1 mM * (+

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Ionic strength is sometimes stated as having units of molal (or molar) and other times stated as being unitless, depending on the book you read. The easiest. - ppt download